
 With shares already reflecting a post-COVID dearth of revenue streams, Pfizer’s growth outlook recently took another hit as it halted development of its experimental weight-loss pill after a high rate of side effects appeared in a mid-stage study.More than half of patients in the study had to stop taking danuglipron due to nausea and vomiting, according to a statement on Friday. While the most common adverse events in the study were mild, nausea was seen in up to 73% of patients, vomiting in up to 47%, and diarrhea in as much as 25%. Pfizer will continue developing a once-daily version of the pill in hopes that it may improve its tolerability profile.Pfizer had hoped to follow Eli Lilly and Novo Nordisk’s lead (both of which provide the shot-based GLP-1 agonists that have made so much news this year) and massive outperformance.
With shares already reflecting a post-COVID dearth of revenue streams, Pfizer’s growth outlook recently took another hit as it halted development of its experimental weight-loss pill after a high rate of side effects appeared in a mid-stage study.More than half of patients in the study had to stop taking danuglipron due to nausea and vomiting, according to a statement on Friday. While the most common adverse events in the study were mild, nausea was seen in up to 73% of patients, vomiting in up to 47%, and diarrhea in as much as 25%. Pfizer will continue developing a once-daily version of the pill in hopes that it may improve its tolerability profile.Pfizer had hoped to follow Eli Lilly and Novo Nordisk’s lead (both of which provide the shot-based GLP-1 agonists that have made so much news this year) and massive outperformance.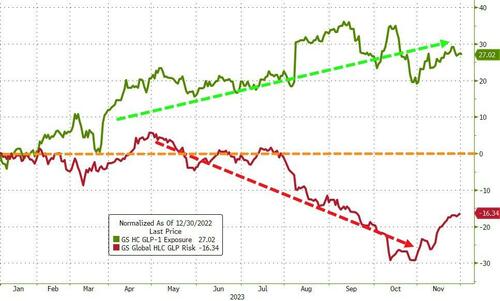 Pfizer and AstraZeneca saw pills as a way to make inroads into a market projected to reach $100 billion within seven years. Maybe not so much now.Pfizer CEO Albert Bourla has said that oral weight-loss drugs will capture a third of the obesity market. The company had pinned its weight-loss hopes on danuglipron after discontinuing another experimental obesity drug in June due to safety concerns.Pfizer shares were down around 4% in the pre-market on Friday, back near their lowest since March 2020 on the back of the news. Pfizer closed Friday’s trading at $28.91.
Pfizer and AstraZeneca saw pills as a way to make inroads into a market projected to reach $100 billion within seven years. Maybe not so much now.Pfizer CEO Albert Bourla has said that oral weight-loss drugs will capture a third of the obesity market. The company had pinned its weight-loss hopes on danuglipron after discontinuing another experimental obesity drug in June due to safety concerns.Pfizer shares were down around 4% in the pre-market on Friday, back near their lowest since March 2020 on the back of the news. Pfizer closed Friday’s trading at $28.91.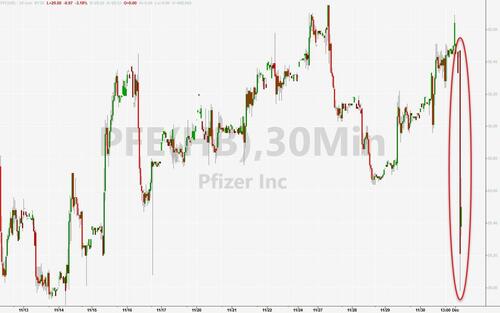 More By This Author:Tiger Global’s Top Venture Fund Suffers 18% Loss Following Portfolio Markdowns
More By This Author:Tiger Global’s Top Venture Fund Suffers 18% Loss Following Portfolio Markdowns
North Korea & Eritrea Dominate The Global Hotspots Of Modern Slavery
Chrysler Building’s EU Co-Owner Goes Bankrupt
















